Decode VRL
Learn more about the study objectives and vitreoretinal lymphoma
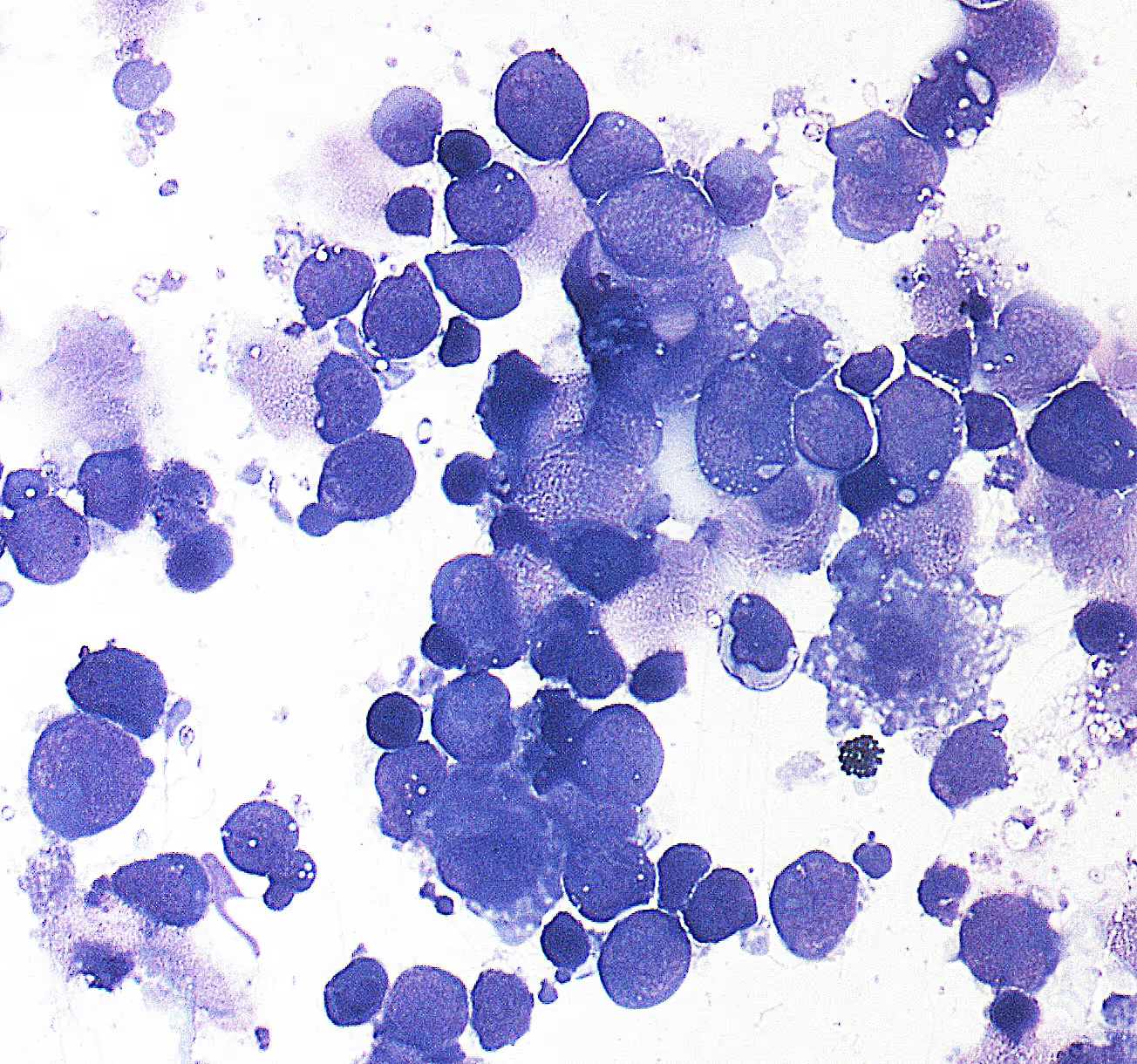
Vitreoretinal lymphoma (VRL) is a rare and aggressive type of B-cell lymphoma that typically affects the inside of the eye (vitreoretinal space) and is often associated with primary CNS lymphoma (PCNSL). The disease, which usually occurs in older adults, is associated with a low survival rate, particularly when diagnosis is delayed. Due to the rarity of VRL and the often unspecific symptoms that clinically overlap with inflammatory eye diseases, VRL is often detected late, which makes early treatment difficult and impairs prognosis.
VRL is difficult to diagnose because the symptoms are often similar to those of uveitis, an inflammation of the middle skin of the eye. Patients often complain of blurred vision and eye problems, which initially present as inflammation during routine examinations. Standard diagnoses such as optical coherence tomography (OCT) and fluorescence angiography cannot reliably differentiate inflammatory and neoplastic diseases of the eye. In addition, the extraction of diagnostic material is complex, as tissue removal from the vitreous body (vitrectomy) is an invasive method and often only supplies small amounts of cells, which reduces diagnostic sensitivity.


The DECODE VRL study is necessary because current diagnostic methods offer only limited accuracy and the diagnosis of VRL is often delayed by months to years. The previous diagnostic chain for VRL comprises several steps, all of which involve difficulties and potential sources of error.
Cytological examination of the vitreous body, in which cells are examined for neoplastic changes, is often inaccurate because VRL cells have degenerative changes and sensitivity is low. There is also a lack of standardized protocols for molecular biological investigations, which leads to widely varying results in different clinics.
Targeted analysis of genetic changes:
VRL is seen as a variant of the diffuse large CNS B-cell lymphoma (PCNSL), which is genetically part of the MCD/cluster-5 subgroup of DLBCL. A specifically developed gene panel including copy number changes, mutations, and miRNAs (19b, 21 and 92) enables highly sensitive and specific diagnostics. In addition, risk factors for secondary CNS involvement will be analysed.
Evaluation of the “liquid biopsy”:
The investigation of cell-free DNA from the vitreous body or an anterior chamber aspirate has high potential for minimally invasive molecular diagnostics, in particular for follow-up studies, and is therefore being evaluated in parallel as part of the study on the supernatant of the vitrectomy preparation.
Next generation sequencing (NGS) -based clonality analysis:
Conventional analysis of B-cell clonality shows limited sensitivity and specificity due to somatic hypermutation of immunoglobulin genes and the immuno-privileged status of the inner eye. The NGS-based analysis of the clonality of B cells, which has been significantly promoted by our working group, enables precise quantification of clonal rearrangements and allows the malignant clone to be followed as it progresses. This method has higher sensitivity and specificity and is particularly suitable for low-cell samples, such as those often found in vitrectomy.

The DECODE VRL study is a diagnostic study that is structured as a prospective, multicenter study and in which up to 20 specialized eye clinics in Germany participate as sampling centers. The study has a planned duration of a total of six years and comprises a four-year recruitment phase and a two-year follow-up phase of patients. The most important steps in the study process include:
The DECODE VRL study will not only contribute to improving diagnostics, but will also create a platform for future research and clinical trials. A central biobank, in which remaining vitreous samples are stored, will be available for further research purposes. This enables scientists to research new biomarkers and therapeutic approaches. In the long term, the network established by the DECODE VRL study will serve as a basis for multicenter clinical trials and innovative therapies for VRL and other eye tumors.
By integrating a national registry, clinical data on VRL patients is also collected and analyzed, which could provide valuable insights into the epidemiological and clinical course of the disease.
1: Elbert M, Neumann F, Kiefer M, Christofyllakis K, Balensiefer B, Kos I,Carbon G, Kaddu-Mulindwa D, Bittenbring JT, Fadle N, Regitz E, Fend F, BonzheimI, Thurner L, Bewarder M. Hyper-N-glycosylated SEL1L3 as auto-antigenic B-cellreceptor target of primary vitreoretinal lymphomas. Sci Rep. 2024 Apr26;14(1):9571. doi: 10.1038/s41598-024-60169-5. PMID: 38671086;
2: Bonzheim I, Salmerón-Villalobos J, Süsskind D, Szurman P, Gekeler F, SpitzerMS, Salaverria I, Campo E, Coupland SE, Quintanilla-Martinez L, Fend F.Molekulare Diagnostik des vitreoretinalen Lymphoms [Molecular diagnostics forvitreoretinal lymphoma]. Pathologie (Heidelb). 2023 Dec;44(Suppl 3):150-154.German. doi: 10.1007/s00292-023-01251-z. Epub 2023 Nov 10. PMID: 37947807.
3: Schiemenz C, Lüken S, Klassen AM, Ranjbar M, Illerhaus G, Fend F, Heindl LM,Chronopoulos A, Grisanti S, Kakkassery V. Klinisches Vorgehen bei intraokulärenLymphomen [Clinical procedures for intraocular lymphomas]. Ophthalmologie. 2022Jul;119(7):675-685. German. doi: 10.1007/s00347-022-01651-1. Epub 2022 Jun 9.PMID: 35925411.
4: Fend F, Bonzheim I, Kakkassery V, Heindl LM, Illerhaus G. Lymphome des Augesund seiner Adnexe : Moderne pathologische Diagnostik und systemische Therapie[Lymphoma of the eye and its adnexa : Modern pathological diagnostics andsystemic treatment]. Ophthalmologie. 2022 Jul;119(7):664-674. German. doi:10.1007/s00347-022-01650-2. Epub 2022 May 23. PMID: 35925409.
5: Bonzheim I, Sander P, Salmerón-Villalobos J, Süsskind D, Szurman P, GekelerF, Spitzer MS, Steinhilber J, Kohler E, Büssgen M, Schittenhelm J, Salaverria I,Campo E, Coupland SE, Quintanilla-Martinez L, Fend F. The molecular hallmarks ofprimary and secondary vitreoretinal lymphoma. Blood Adv. 2022 Mar 8;6(5):1598-1607. doi: 10.1182/bloodadvances.2021004212. PMID: 34448823; PMCID:PMC8905692.
6: Sobolewska B, Chee SP, Zaguia F, Goldstein DA, Smith JR, Fend F, Mochizuki M, Zierhut M. Vitreoretinal Lymphoma. Cancers (Basel). 2021 Aug 4;13(16):3921. doi: 10.3390/cancers13163921. PMID: 34439078; PMCID: PMC8394064.
7: Kakkassery V, Jünemann AM, Bechrakis NE, Grisanti S, Ranjbar M, Zschoche M, Heindl LM. Lymphom am Auge: Präzise Diagnostik und Klassifikation als Schlüssel einer erfolgreichen personalisierten Therapie [Ocular lymphoma : Precise diagnostics and classification as key for successful personalized treatment]. Ophthalmologe. 2020 Jun;117(6):499-507. German. doi: 10.1007/s00347-019-01020-5. PMID: 31811368.
8: Fend F, Süsskind D, Deuter C, Coupland SE. Maligne Lymphome des Auges[Malignant lymphomas of the eye]. Pathologe. 2017 Nov;38(6):515-520. German.doi: 10.1007/s00292-017-0378-6. PMID: 28993856.
9: Kakkassery V, Schroers R, Coupland SE, Wunderlich MI, Schargus M, Heinz C, Wasmuth S, Heiligenhaus A, Ahle G, Lenoble P, Schlegel U, Schmiegel W, Dick HB, Baraniskin A. Vitreous microRNA levels as diagnostic biomarkers for vitreoretinal lymphoma. Blood. 2017 Jun 8;129(23):3130-3133. doi: 10.1182/blood-2017-01-765180. PMID: 28389463.
10: Fend F, Ferreri AJ, Coupland SE. How we diagnose and treat vitreoretinallymphoma. Br J Haematol. 2016 Jun;173(5):680-92. doi: 10.1111/bjh.14025. Epub2016 May 2. Erratum in: Br J Haematol. 2018 Jun;181(5):712. doi:10.1111/bjh.15395. PMID: 27133587.
11: Bonzheim I, Giese S, Deuter C, Süsskind D, Zierhut M, Waizel M, Szurman P,Federmann B, Schmidt J, Quintanilla-Martinez L, Coupland SE, Bartz-Schmidt KU,Fend F. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: avaluable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015 Jul2;126(1):76-9. doi: 10.1182/blood-2015-01-620518. Epub 2015 Apr 21. PMID:25900979.
12: van den Brand M, Möbs M, Otto F, Kroeze LI, Gonzalez de Castro D,Stamatopoulos K, Davi F, Bravetti C, Kolijn PM, Vlachonikola E, Stewart JP, PottC, Hummel M, Darzentas N, Langerak AW, Fend F, Groenen PJTA. EuroClonality-NGSRecommendations for Evaluation of B-Cell Clonality Analysis by Next-GenerationSequencing: A Structured Approach with the DEPART Algorithm. J Mol Diagn. 2023Oct;25(10):729-739. doi: 10.1016/j.jmoldx.2023.06.011. Epub 2023 Jul 17. PMID:37467928.
13: van den Brand M, Rijntjes J, Möbs M, Steinhilber J, van der Klift MY, HeezenKC, Kroeze LI, Reigl T, Porc J, Darzentas N, Luijks JACW, Scheijen B, Davi F,ElDaly H, Liu H, Anagnostopoulos I, Hummel M, Fend F, Langerak AW, Groenen PJTA; EuroClonality-NGS Working Group. Next-Generation Sequencing-Based Clonality Assessment of Ig Gene Rearrangements: A Multicenter Validation Study byEuroClonality-NGS. J Mol Diagn. 2021 Sep;23(9):1105-1115. doi:10.1016/j.jmoldx.2021.06.005. Epub 2021 Jun 26. PMID: 34186174.
14: Scheijen B, Meijers RWJ, Rijntjes J, van der Klift MY, Möbs M, Steinhilber J,Reigl T, van den Brand M, Kotrová M, Ritter JM, Catherwood MA, Stamatopoulos K,Brüggemann M, Davi F, Darzentas N, Pott C, Fend F, Hummel M, Langerak AW,Groenen PJTA; EuroClonality-NGS Working Group. Next-generation sequencing ofimmunoglobulin gene rearrangements for clonality assessment: a technicalfeasibility study by EuroClonality-NGS. Leukemia. 2019 Sep;33(9):2227-2240. doi:10.1038/s41375-019-0508-7. Epub 2019 Jun 13. PMID: 31197258
Whether as a patient, relative, medical professional colleague or supporter: Your involvement and interest are decisive for the success of the DECODE VRL study.
Contact us if you have any questions about the study, participation or the scientific background.
Liv Dollmann
Study coordination
Department of Ophthalmology, University Hospital Schleswig-Holstein
Lübeck Campus
Phone: +49 451 500-43911
email: liv.dollmann@uksh.de
Working together for better diagnosis and treatment of vitreoretinal lymphoma.
“Your support brings us one step closer to deciphering lymphoma in the eye”
